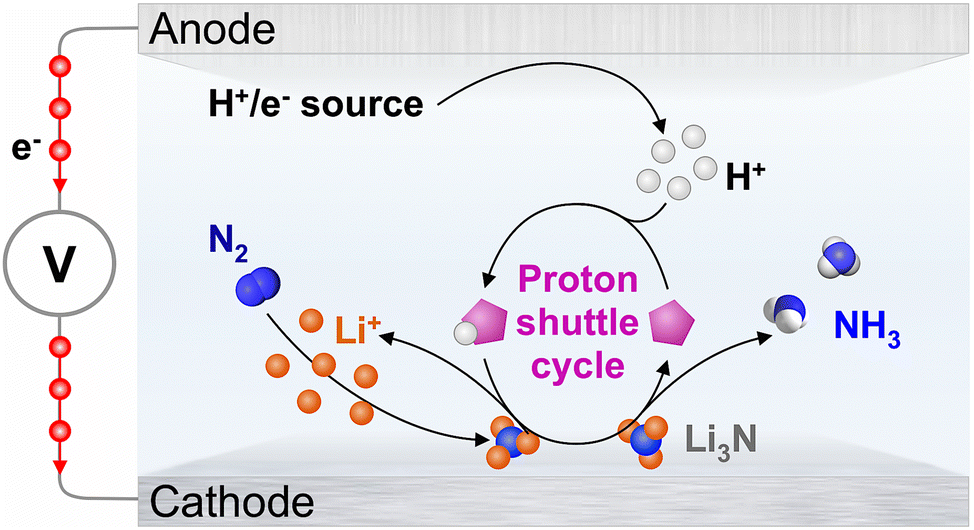
Sustainable ammonia production from renewable energy sources is an ongoing challenge, but electrochemical lithium-mediated nitrogen reduction can offer a scalable solution. In this process, an electrolyte is used to transport hydrogen ions from the anode to the cathode, where they react with nitrogen gas to form ammonia. However, traditional carriers of hydrogen ions, like ethanol, are not sustainable over the long term.
In a recent study, researchers from Monash University, led by Prof. Douglas R. MacFarlane (my former mentor) and Dr. Alexandr (Sasha) Simonov investigated ethanol’s degradation mechanism and explored alternatives. Using a top-performing electrolyte, they found that iso-propanol can sustain ammonia electrosynthesis at close to 100% faradaic efficiency without being significantly converted to side-products. The optimal chemical nature and concentration of the proton carrier are unique for every set of reaction conditions, and C2–C4 alcohols and phosphonium cations present the most viable candidates as effective proton carriers for the Li-NRR under the conditions currently examined in the field. The study also showed that electrooxidation of tetrahydrofuran, which can induce irreversible degradation of ethanol, might be avoided if hydrogen gas is introduced as a proton/electron source, but this presents its own challenges. The findings of this study provide important insights into the development of sustainable ammonia electrosynthesis technologies.
The results were published in Energy Environ. Sci., and can be found here: https://doi.org/10.1039/D2EE03901J