A promising method for the electrochemical synthesis of ammonia from nitrogen and hydrogen has been discovered in new research, which could decentralise the current mass production of fertiliser. The method involves lithium-ion cathodic reduction in an organic solvent electrolyte, followed by lithium-nitrogen reaction. Conventional hydrogen oxidation catalysts for the complementary anodic process, on the other hand, are unstable in these conditions. Fu et al. discovered that a gold-platinum alloy can robustly catalyse this oxidation and thus steadily produce ammonia protons under continuous flow conditions.
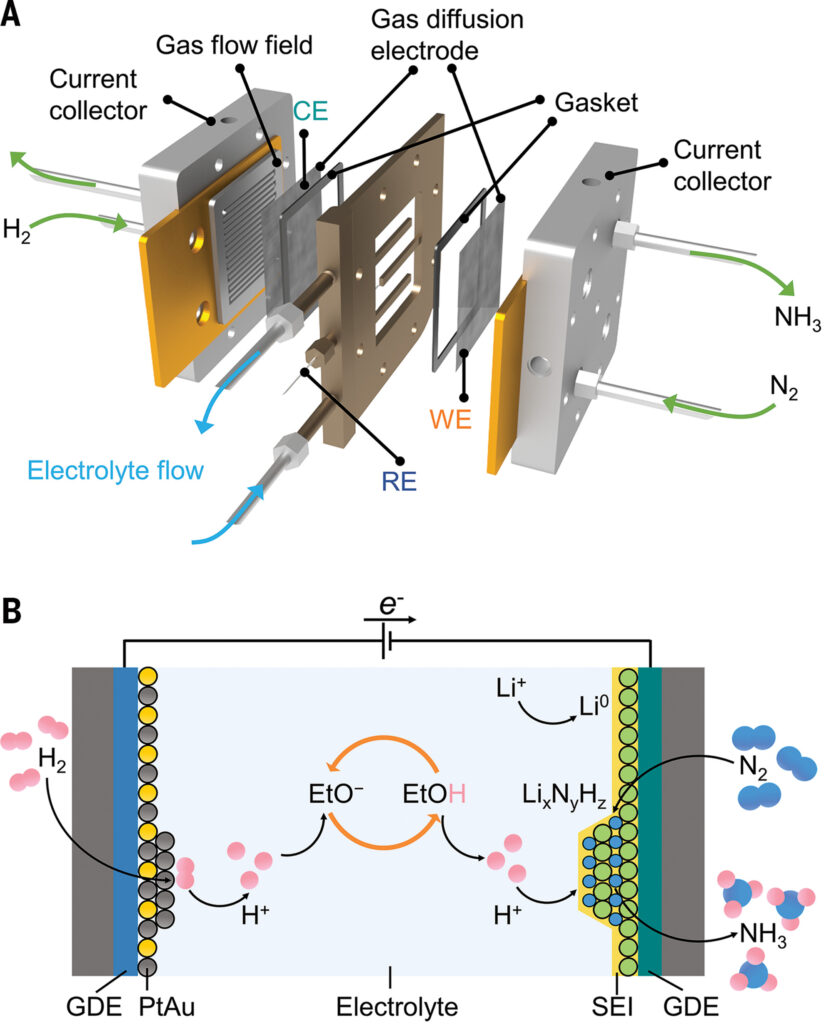
Ammonia is a necessary component of fertilisers, pharmaceuticals, and fine chemicals, as well as an excellent carbon-free fuel. At ambient temperatures, the process of lithium-mediated nitrogen reduction has shown great promise for electrochemical ammonia synthesis. The researchers built a three-compartment continuous-flow reactor with a solid-state composite-based gas diffusion electrode (GDE) sandwiched between a gas flow field and an electrolyte chamber, allowing gaseous reactants to be fed directly to one side of the electrode while the electrolyte was fed on the other.
The method works by diffusing lithium ions from the bulk electrolyte through the interphase of the solid-electrolyte. On the electrode, the ions are electrochemically reduced into metallic lithium, which then reacts with nitrogen to form lithium nitride. This is then protonated by a proton shuttle (EtOH) to continuously release ammonia. The anode’s hydrogen oxidation provides a sustainable source of protons. The working and counter electrodes were solid-state composites with varying pore sizes, as well as PtAu catalysts on a solid-state composite substrate. A platinum wire served as the pseudo-reference electrode.
The experiment discovered that platinum was not stable for hydrogen oxidation in the organic electrolyte, but a platinum-gold alloy lowered the anode potential and avoided the organic electrolyte’s decremental decomposition. The continuous-flow electrolyzer equipped with 25-square centimeter-effective area gas diffusion electrodes achieved a faradaic efficiency for ammonia production of up to 61 ± 1% and an energy efficiency of 13 ± 1% at a current density of -6 milliamperes per square centimetre.
This method is advantageous because it eliminates the need for a sacrificial solvent as a proton source and allows for lower cell voltage to improve ammonia selectivity and energy efficiency. The findings of the researchers suggest that this approach could decentralise current mass fertiliser production and allow for a more sustainable and environmentally friendly method of ammonia synthesis.
This research was published in SCIENCE, 16 Feb 2023, Vol 379, Issue 6633 pp. 707-712 and can be found here: DOI:10.1126/science.adf4403