Electrocatalysis is becoming more complex, making it challenging to explore. With so many reports published annually, researchers frequently make electrocatalytic systems more challenging to improve performance. Current electrochemical and analytical methods need help to meet this complexity, making fundamental catalytic aspects elusive. This leads to the development of complex interface-related hypotheses. A pathway with essential electrocatalytic and analytical points must be addressed before meaningful beginning models, and new hypotheses are raised. This path includes determining if the activity changed independently, showing the in-situ composition, and figuring out the reaction mechanism and active sites. A new article by J. Niklas Hausmann and Prashanth W. Menezes in “Applied Catalysis B: Environmental” helps researchers, readers, and reviewers understand how complicated electrocatalysis is. It also gives a methodical way to see if basic issues are covered before complicated hypotheses that could be wrong are put forward.
It is hard to figure out what makes electrocatalysis work because the systems that do it are so complicated, like electrodes with different shapes and sizes. The way surface area and active site determination are done now makes it hard to figure out the intrinsic activity of simple systems. This leads to a mismatch between the complexity of the system, how it is described, and how it works in theory. The perspective provides solutions to these challenges and improves the understanding of electrocatalysis.
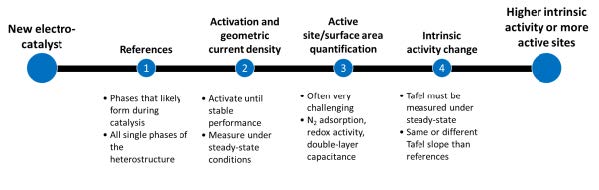
The study focuses on determining the intrinsic activity of a new electrocatalyst compared to reference samples. Four points are suggested: choosing suitable references, determining geometric current density, quantifying the number of active sites, and determining the surface area. References should be simple, widely applied compounds with the same functional elements as the catalyst. The study suggests that most newly reported systems enhance activity by exposing more active sites of a known catalyst. The number of active sites or surface area can be determined by applying a potential above and below a redox transition or by measuring the surface. However, these methods are often challenging and ambiguous, and there is no straightforward way to determine the number of active sites or surface area of electrocatalysts. Individual solutions for each system are required, likely involving more than one method.
The other point in electrocatalysis is determining intrinsic activity parameters, such as turnover frequency. The Tafel slope, which depends on the catalyst’s mechanism, is often used to determine if two catalysts operate with the same exact mechanism and active site. However, potentiodynamic methods often influence Tafel slopes, making them insufficient for mechanistic insights. For the new catalyst, it is important to know the geometric current density at the same potential, the difference in the number of active sites, and how the Tafel slope changes. This data can help determine if the reported catalyst has a higher geometric current density due to an intrinsic change of the active sites or only their number. Faradaic efficiency and product selectivity are crucial aspects of electrocatalysis. The in-situ atomic and macroscopic structure of the electrode is essential for understanding the performance of the catalyst.
We need to look at electrocatalysts in their natural setting, study their atomic and microscopic structure, describe the electrode after catalysis, use in-situ methods, and combine methods to fully understand them. In-situ characterizations provide valuable insights into the active catalyst’s structure, identifying elements leached or mixed phases. In-situ methods can directly investigate the active catalyst but are more challenging. Combining microscopic and spectroscopic methods is essential for conclusiveness, as additional spectroscopic methods must support microscopic findings. Methods can be bulk-sensitive or surface-sensitive, and sample preparation can cause a specific material selection. Combining various analytical methods is necessary to ensure every phase is noticed. Dionigi et al.’s study, for example, looked at how the structure of nickel and cobalt oxyhydroxides with iron changes in alkaline electrolytes during the OER. This showed how they really looked in that environment. This highlights the challenge of obtaining precise in-situ structures in electrocatalysts.
It can be hard to choose useful DFT input models for simulating overpotentials in multiphase catalysts because there are so many phases, surfaces, interfaces, and chemical environments where potential active sites could be. This process can be iterative, with specific sites and mechanisms repeatedly assumed and calculations performed. Finding the active surface or interface, suggesting a reaction mechanism with intermediates, and figuring out the adsorption energies of the assumed intermediates are the four steps shown below. The complexity of these points is significant, as even for simple single-phase systems, the complexity is multiple times larger for heterostructured systems with interfaces. Therefore, hundreds of DFT studies are required to provide a conclusive understanding of novel multiphase catalysts. Many reports need to account for this complexity sufficiently, especially if mainly experimental reports are spiced up with brief DFT investigations.
The complexity of electrocatalysis systems is becoming increasingly challenging due to the dynamic behavior of materials’ surfaces during electrocatalysis. It is hard to figure out how these systems work, especially when catalysis happens in a small electrode area, like a surface, impurity, defect, or interface. The paper shows a way to get useful DFT input models and understand the basic aspects of these kinds of systems. Several things need to be done in order to do this well: finding good references, telling the difference between more intrinsic activity and multiple active sites, fully characterizing the whole system during electrocatalytic operation, and comparing DFT input models in a critical way. The authors provide a potential solution to meet the complexity of electrocatalysis.
The full article can be found here: https://doi.org/10.1016/j.apcatb.2023.123447
Taking the forefront in the movement towards a sustainable energy future through the implementation of eco-friendly ammonia and hydrogen solutions